L-Cysteine, 25 g, CAS No. 52-90-4 | Protected Amino Acids | Amino Acids and Amino Acid Derivatives | Organic & Bioorganic Chemicals | Chemicals | Carl Roth - International
L-Proline, 25 g, CAS No. 147-85-3 | Protected Amino Acids | Amino Acids and Amino Acid Derivatives | Organic & Bioorganic Chemicals | Chemicals | Carl Roth - International
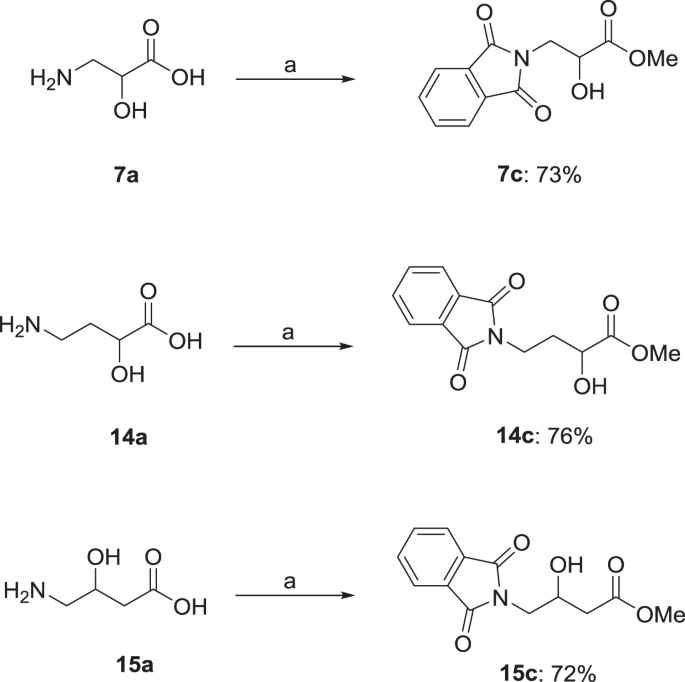
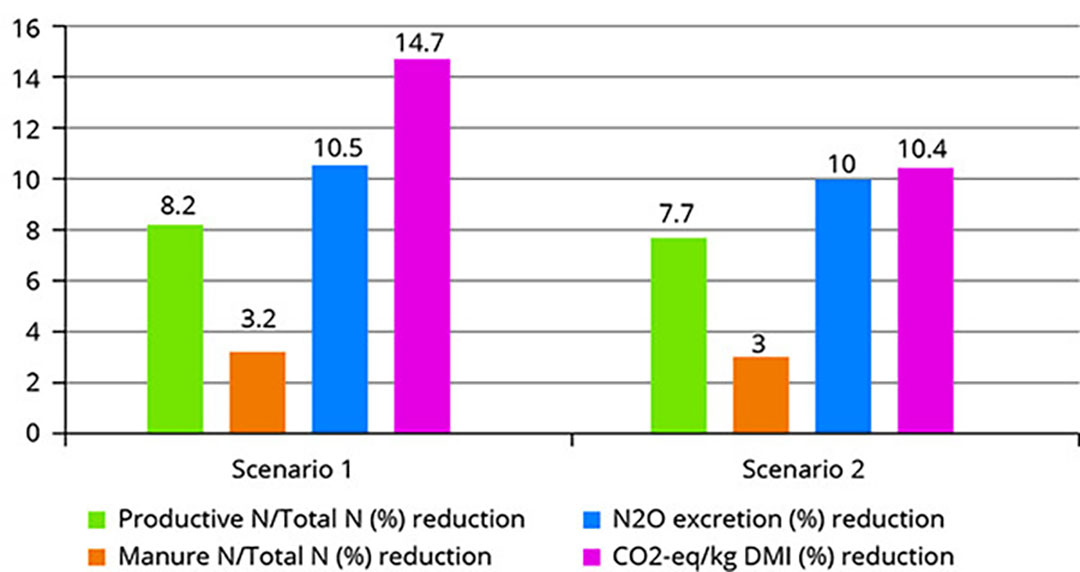
Synthesis and biological evaluation of α- and β-hydroxy substituted amino acid derivatives as potential mGAT1–4 inhibitors | Medicinal Chemistry Research
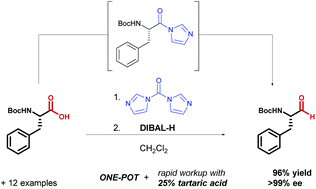
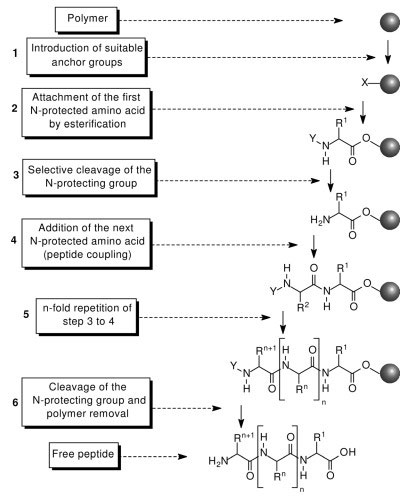
A rapid and efficient one-pot method for the reduction of N-protected α-amino acids to chiral α-amino aldehydes using CDI/DIBAL-H - Organic & Biomolecular Chemistry (RSC Publishing)

Protecting‐Group‐Free Amidation of Amino Acids using Lewis Acid Catalysts - Sabatini - 2018 - Chemistry – A European Journal - Wiley Online Library
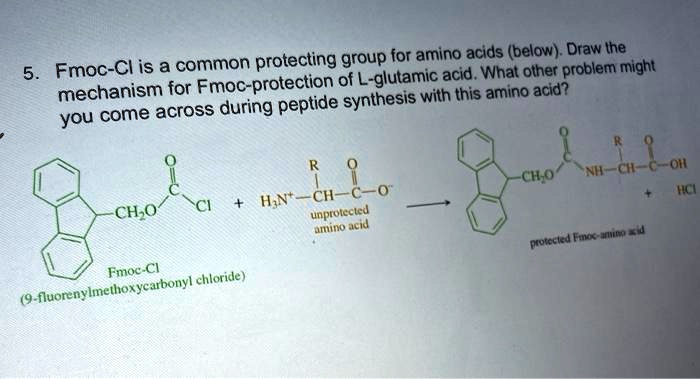
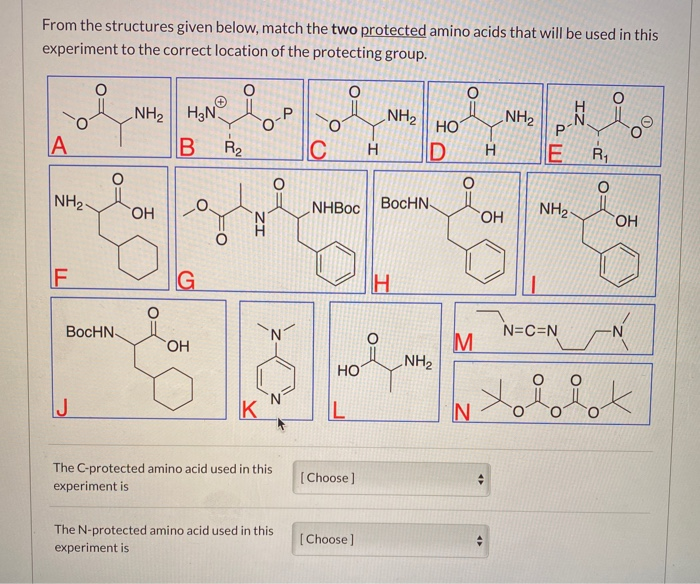
SOLVED: Fmoc-Cl is a common protecting group for amino acids. What other problem might arise with this amino acid during peptide synthesis?
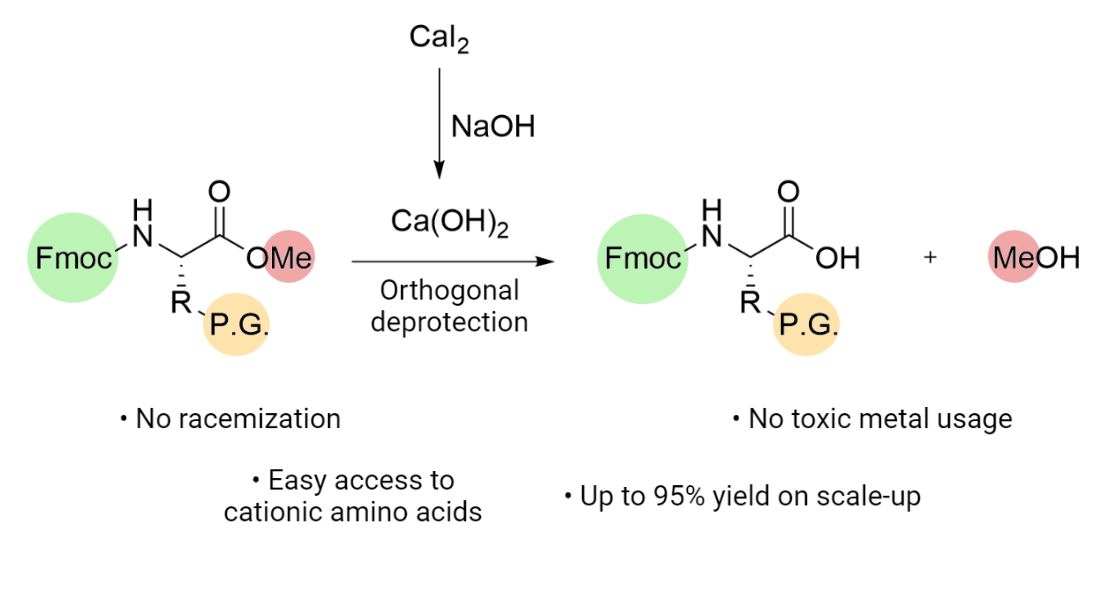
Molecules | Free Full-Text | Efficient Fmoc-Protected Amino Ester Hydrolysis Using Green Calcium(II) Iodide as a Protective Agent
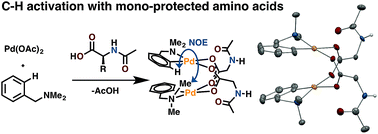
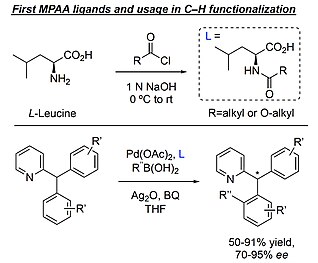
Mono-N-protected amino acid ligands stabilize dimeric palladium(ii) complexes of importance to C–H functionalization - Chemical Science (RSC Publishing)